The First Law of Thermodynamics is an advance version of the conservation of energy, designed for thermodynamics systems. This is mainly used to define the conservation of energy states that the complete energy of the unique system which is completely constant. In this energy can be easily transformed in every forms. This is the completely new and advance law which can be formulated. In our best quality Thermodynamics assignment help, we are trying to define the each and every information which delivers accurate knowledge to the students. We want to deliver the accurate and reliable information to the students with the help of Thermodynamics case study help.
Importance of First Law of Thermodynamics
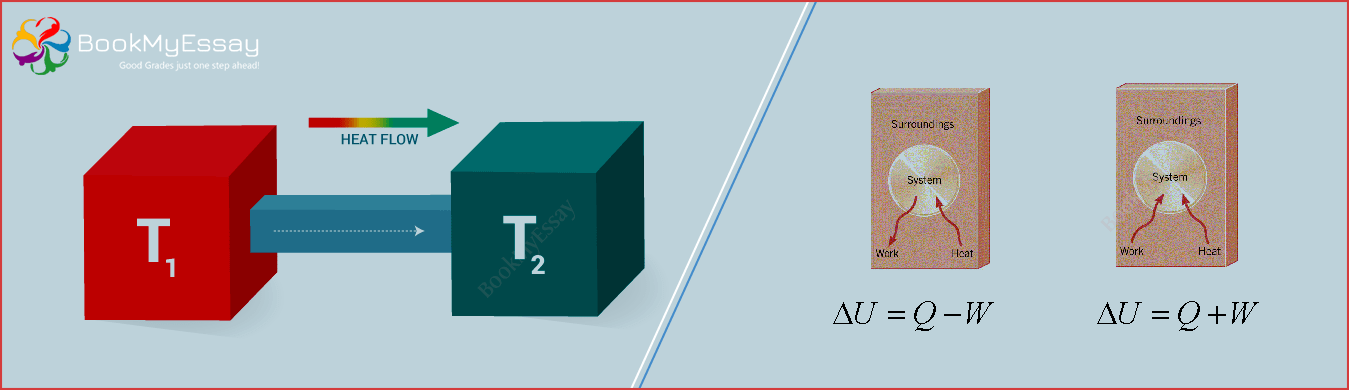
The main fact is that it is one of the important branches of the physics which mainly pacts with the complete relationship among the heat and entire forms of energy. The first law of thermodynamics is one of the important way or you can say that useful principle of the conservations of energy. This mainly defines the complete relationship between the heat and work. The main important information of the fist law is to make the balance among the energy and heat. Here we are trying to deliver the complete information to the students in our Thermodynamics assignment writing help.
- This is mainly used to build the relationship between the heat and work to get the accurate result.
- With the help of this law we get the fixed amount of heat which is required to complete the process. In this law we get the idea how heat you need to complete the entire process.
- This mainly divides the complete concept of the work and heat into equivalent portion.
- This is the main principle of the conservations of energy, which mainly provide the best and useful way to balance the heat and energy to get the significant result.
The first law of thermodynamics always delivers the big result to users because it mainly deals with the total amount of the energy in the universe. It delivers the entire result in different manners which never changes. The energy created by the first law of thermodynamic never change and altered. This is only changed to make another form of transfer from one object to another object. With the help of our best Thermodynamics homework help you get the proper information related to the require topic in appropriate format. With the thermodynamic we never get the electronics products which we are using in our daily life. Because with the help of thermodynamic principles we are making the balance in the form of energy and heat. This balance also helps us to make the new and advance electronics products to human beings. The main fact is that we get the energy with the help of thermodynamic because it is also necessary to live.





